
Chlamydia trachomatis/Neisseria gonorrhoeae (CT/NG) – Nucleic Acid Amplification Testing (NAAT)
Conformément au Règlement de l’Ontario 671/92 de la Loi sur les services en français, les renseignements d’analyses de laboratoire liés à la présente page ne sont offerts qu’en anglais parce qu’ils sont de nature scientifique ou technique et destinés uniquement à l’usage des fournisseurs de soins de santé qualifiés et non aux membres du public.
Background
This page provides nucleic acid amplification testing (NAAT) information for Chlamydia trachomatis (CT)/Neisseria gonorrhoeae (NG) at Public Health Ontario (PHO). For information regarding other testing options, refer to the following PHO webpages:
Updates
- Recommended number of days after completion of treatment adjusted for Neisseria gonorrhoeae infection test of cure.
Testing Indications
Nucleic acid amplification testing (NAAT) is the recommended method for initial screening or testing for Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG) infections.
Neisseria gonorrhoeae (NG) culture is recommended plus NAAT when suspecting antimicrobial resistance, test of cure, symptomatic patients, pelvic inflammatory disease (PID), pregnancy, and sexual abuse/sexual assault.
First-void urine is the specimen of choice for males. A vaginal swab is the specimen of choice for females. In women who have had a hysterectomy, collect a first void urine for NAAT or a vaginal swab for culture or NAAT. Refer to the Specimen Collection and Handling section for additional acceptable specimen types.
Rectal and/or pharyngeal testing is recommended for individuals who have had unprotected sexual exposures at these sites and are in specific at-risk groups or have risk factors, including:
- gay, bisexual, and men who have sex with men, including trans women;
- individuals engaged in sex work or have had sexual contact with someone engaging in sex work;
- individuals who are known contacts of those infected with CT or NG;
- individuals who have signs or symptoms of rectal or pharyngeal infection.
Rectal and/or pharyngeal testing in individuals who have had exposures at these sites and are not in specific risk groups above may be considered in individual circumstances based on clinical evaluation or local epidemiology.
Rectal bacterial sexually transmitted infections, including CT and NG, have been associated with increased risk of HIV infection in gay, bisexual, and other men who have sex with men, and transgender women. Screening for HIV is highly recommended in these individuals. Details about HIV serology testing at PHO can be found on the HIV Serology Test Information Sheet. Consider initiation of Pre-Exposure Prophylaxis (PrEP) for HIV-negative individuals. For more information on PrEP, refer to ontarioprep.ca.
Acceptance/Rejection Criteria
The following are rejected for testing:
- Specimens for donor screening: Donor testing is not available through PHO. Specimens from patients being screened as potential donors (e.g. organ, tissue, cells, fertility, etc.) should be referred to a laboratory that performs donor screening assays. Specimens received for donor screening at PHO’s laboratory will be rejected.
- Specimens received in an expired kit
- Genital swab specimens collected from female patients that have used vaginal moisturizers (e.g. Replens®)
- Urine specimen volumes outside of collection guidelines: The Roche cobas® PCR Urine Sample Kits serve as a nucleic acid stabilizing transport and storage medium for urine specimens. The transferred urine must be between the black fill lines for accurate testing. Inadequate volumes (i.e. insufficient or excess) will be cancelled.
- Excessively bloody specimens:
- Bloody urines: If there is an excess of blood in the urine specimen (i.e. >35%; specimen will be dark red or brown in colour), it should be discarded and recollected when appropriate. If received at the laboratory, these specimens will be cancelled.
- Bloody swabs: If there is an excess of blood in the swab specimen (i.e. >5%; specimen will have a red or brown colour) it should be discarded and recollected. If received at the laboratory, these specimens will be cancelled. An exception can be made for cases of sexual assault/abuse where recollection is not possible. These will be reviewed on a case by case basis. An indication of sexual assault/abuse needs to be on the requisition for this to be considered.
- Specimen grossly contaminated with feces
- Sample type collected using inappropriate kit/swabs: Refer to Specimen Requirements Table under Specimen Collection and Handling for appropriate kits /swabs.
- Swab collection kits without swab or containing two swabs
- Male urethral and penile meatal swabs: Using the Roche cobas® PCR Media Dual Swab Sample Kit, the only validated sites for this testing are endocervical, vaginal, rectal and pharyngeal swabs. All other sites, including urethral and penile meatal swabs, have not been validated and will be cancelled.
- Rectal swab collected with anal lubricants: Anal lubricants are not recommended for specimen collection as these may cause test interference and these specimens may be cancelled.
Test of Cure (TOC):
General guidelines for NG and CT Test of Cure (TOC) are described below. Refer to the PHAC Canadian Guidelines on Sexually Transmitted Infections for detailed information.
-
For NG: TOC is recommended for all positive sites in all cases of gonorrhea. Culture is the preferred method for TOC and should be performed at least 3 days after completion of treatment. If culture is not available, TOC by NAAT is acceptable and should be performed 3-4 weeks after completion of treatment. Repeat screening is recommended 6 months post-treatment for all individuals with NG infection.
-
For CT: TOC by NAAT is recommended 3 weeks after completion of treatment when compliance to treatment is suboptimal, an alternative treatment regimen is used, for those with persisting signs or symptoms post-treatment, or the individual is pre-pubertal or pregnant. For patients with LGV, follow individuals until CT TOC is negative and symptoms have resolved. In rare circumstances, CT DNA may persist for longer than 4 weeks and therefore must be considered when interpreting positive TOC results. Repeat screening is recommended 3 months post-treatment for all individuals with CT infection.
Specimen Collection and Handling
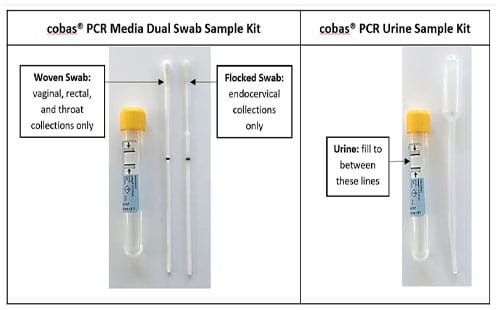
Detailed specimen collection instructions using the Roche cobas® CT/NG kits can be found here: Roche Educational Resources.
Specimen Requirements
| Test Requested | Required Requisition(s) | Specimen Type | Minimum Volume | Collection Kit |
Chlamydia trachomatis/ Neisseria gonorrhoeae (CT/NG) NAAT |
First-void urine (see submission and collection note #4) |
Transfer first-void urine from a sterile container to the Roche cobas® PCR Urine Sample Kit until the fluid level is between the two black fill lines. |
Roche cobas® Urine Sample Kit (Item # 300316) |
|
Chlamydia trachomatis/ Neisseria gonorrhoeae (CT/NG) NAAT |
Endocervical (see submission and collection note #5, #6 ) |
Flocked swab suspended in the collection kit fluid |
Roche cobas® PCR Media Dual Swab Sample Kit (kit order # 300317) |
|
Chlamydia trachomatis/ Neisseria gonorrhoeae (CT/NG) NAAT |
Clinician or patient-collected specimens in a clinical setting
(see submission and collection note #5 , #7, #8) |
Woven swab suspended in the collection kit fluid |
Roche cobas® PCR Media Dual Swab Sample Kit (kit order # 300317) |
Submission and Collection Notes
- Test(s) requests and indications for testing/clinical diagnosis
- Patient setting/population/source
- Patient ‘s first and last name, date of birth, health card number
- Specimen Source and Date of Collection
- Physician name and address
Label the specimen container(s) or collection kit with the patient’s first and last name, date of collection, specimen site/source and one other unique identifier such as the patient’s date of birth or Health Card Number. For additional information see: Criteria for Acceptance of Patient Specimens. Failure to provide this information may result in rejection or testing delay.
The transport media contained in the swab collection kit is in the optimal volume required for accurate testing and should not be adjusted. If the collection kit tube contents are spilled, discard and replace with a new Roche cobas PCR Media Dual Swab Sample Kit or PCR Urine Kit. Failure to use a new kit may invalidate test results. Clean spills with a suitable detergent and water. Do not use sodium hypochlorite (bleach) or other highly reactive reagents, or a noxious gas could be released.
- Prior to sampling, the patient should not have urinated for at least one hour. Female patients must not clean the labial area before collection.
- Collect approximately 10-50 mL of first-void urine in a sterile container.
- Using the provided disposable pipette, transfer urine from the sterile container to the Roche cobas PCR Urine Sample Kit until the fluid level is between the two black fill lines.
- Tightly re- cap the Roche cobas® PCR Urine sample tube, and invert 5 times to mix. Urine specimens must be transferred into the Roche cobas® PCR Urine sample tube immediately for stabilization.
- If specimens cannot be transferred immediately, they can be stored at 2-30°C for up to 24 hours.
Roche cobas PCR Media Dual Swab Sample Kit:
- Contains two swabs – flocked and woven. Refer to Specimen Requirements on which type of swab is suitable for a specimen type.
- Do not pre-wet swabs in Roche cobas PCR Media or any other liquid before collection of any specimens. Primary swab specimen tubes with no swabs or with two swabs have not been collected according to the collection instructions and therefore will not be tested.
- For either swab type, lower the swab specimen into the tube until the visible scoreline on the swab is aligned with the tube rim. The tip of the swab should be just above and not submerged into the liquid prior to breaking the shaft at the dark scoreline. Discard the top portion of the swab. Specimens received with no swab in the sample kit will be cancelled.
Endocervical swab collection: Mucous-free specimens are required for optimal test performance; the presence of mucous may cause process delays. For endocervical specimen collection, first use the large woven swab provided in the Roche cobas® PCR Media Dual Swab Sample Kit or equivalent device to clean and remove excess mucous.
Vaginal, rectal, pharyngeal swab collection: Specimens should be collected using the woven swab. Do not let the woven swab touch any surface before placing it into the sample kit. Do not use the flocked swab; these specimens will be cancelled. Minimize use of over-the-counter hygiene and/or prescription products in the rectum or throat during or prior to specimen collection to avoid the potential for test interference. Swabs collected by a clinician or patient in a clinical setting are acceptable. For at-home self-collection, only rectal and pharyngeal swabs have been validated.
Urogenital specimens collection: Products containing carbomer(s), including vaginal lubricants, speculum jellies, creams and gels may interfere with the test and should not be used during or prior to collecting urogenital specimens.
Limitations
- Medico-legal investigations: CT and NG culture is the preferred and recommended method for medico-legal investigations; however, specimens submitted for NAAT will also be accepted. A positive NAAT result will be confirmed by another NAAT using a different set of primers as per the current Public Health Agency of Canada (PHAC) Canadian Guidelines on Sexually Transmitted Infections. Specimens received from patients <14 years of age have not been validated by the manufacturer; however, they will be tested by PHO with a disclaimer added.
- Patient-collected specimen collection is not designed to replace cervical exams and endocervical specimens for diagnosis of female urogenital infections in women. Patients may have cervicitis, urethritis, urinary tract infections, or vaginal infections due to other causes or concurrent infections with other agents.
- Women who have symptoms of pelvic inflammatory disease (PID) should not use self-collected vaginal swab specimens as a replacement for a pelvic exam.
Storage and Transport
Specimens should be stored at 2-30°C following collection and shipped to PHO’s laboratory. Specimens in an unexpired Roche cobas® PCR Media are stable within 12 months of collection; however, should be transported to PHO’s laboratory shortly after collection. All clinical specimens must be shipped in accordance to the Transportation of Dangerous Good Act.
Test Frequency and Turnaround Time (TAT)
Chlamydia trachomatis (CT)/Neisseria gonorrhoeae (NG) NAAT is performed:
- Daily, Monday to Saturday, at PHO’s laboratory, Toronto site
- Daily, Monday to Friday, at PHO’s laboratory, Ottawa site
Turnaround time is up to 3 business days from receipt at the PHO’s laboratory.
Chlamydia trachomatis (CT)/Neisseria gonorrhoeae (NG) NAAT is performed using the Roche cobas CT/NG Assay, a duplex real-time PCR assay for the qualitative detection of CT and NG. Simultaneous testing of both CT and NG occurs from a single specimen (i.e., one collection kit). Testing for CT or NG as a single test request by NAAT is not available and requests will be tested with the CT/NG duplex assay.
Algorithm
Screening is performed on all specimens with the duplex CT/NG NAAT. Confirmatory testing is performed on specimens that test positive, in the following situations:
- NG confirmatory testing: Performed on NG-positive specimens for extragenital sites, children <12 years of age, cases of sexual abuse/sexual assault, and medico-legal investigations. Confirmatory testing for NG is performed using the Roche cobas® omni Utility Channel with the PivNG Assay V2 (IDT). This assay is not currently approved by Health Canada but has been validated for use at PHO.
- CT confirmatory testing: Performed on CT-positive specimens for children <12 years of age, cases of sexual abuse/sexual assault, and medico-legal investigations. CT confirmatory testing is performed using the Cepheid Xpert CT assay.
- Lymphogranuloma venereum (LGV) testing: Rectal swab specimens positive for CT from males, trans-female and/or individuals identifying as male at birth will be routinely forwarded to the National Microbiology Laboratory (NML) for LGV testing. Gender identity is mandatory information for LGV testing. Specimens positive for CT from other sources will only be sent if LGV testing is specifically requested. Refer to the LGV Test Information Sheet for more information.
Interpretation
CT and NG are interpreted and reported individually as Detected or Not Detected.
The following table provides possible test results with associated interpretations:
| Result |
Interpretation |
Comments |
|---|---|---|
|
Negative |
CT and/or NG Not Detected |
|
|
Positive |
CT and/or NG Detected |
|
|
Inconclusive |
CT and/or NG Inconclusive |
Repeat testing for if clinically indicated. |
|
Invalid |
Invalid |
An invalid result may be due to interfering substances or suboptimal content in the specimen. Repeat testing if clinically indicated. |
Reporting
Results are reported to the physician, authorized health care provider (General O. Reg 45/22, s.18) or submitter as indicated on the requisition.
Critical positive reports, including specimens from pregnant patients, pre-pubertal children (<12 years old) and medico-legal specimens (pre-pubertal children, sexual abuse in peri-pubertal and pre-pubertal children, sexual assault and legal cases) are phoned and faxed if the client is registered for auto fax.
Other positives are only faxed if client is registered for auto fax.
Specimens that are positive for CT or NG are reported to the Medical Officer of Health as per the Ontario Health Protection and Promotion Act.
References
- Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG)
- cobas® CT/NG, Qualitative nucleic acid test for use on the cobas® 6800/8800 Systems, Package Insert 08978905001-01EN. Doc Rev 1.0. 05/2019
- cobas® CT/NG, Qualitative nucleic acid test for use on the cobas® 6800/8800 Systems, Package Insert 07997981001-03EN. Doc Rev 3.0. 11/2021
Vous n'avez pas de compte MonSPO? S'inscrire maintenant

