
Serology Testing – Laboratory Specimen Collection and Submission Instructions
Consistent with O. Reg. 671/92 of the French Language Services Act, test ordering and instructions on this page is only available in English because it is scientific or technical in nature and is for use only by qualified health care providers and not by members of the public.
Public Health Ontario conducts serology testing for a number of different diseases. This page provides specimen collection and submission information for serology tests performed at PHO to enhance specimen processing and turnaround times.
For detailed instructions for requesting laboratory tests from PHO, please visit the Test Information Index and navigate to a specific disease.
Required Number of Tubes Submitted per Patient
Generally only one full draw 5 mL Serum Separator Tube (SST) is needed for testing. Submission of less than a full draw could lead to incomplete testing depending on the test(s) requested. If several tests are requested, two or more tubes may be required. The list below outlines the most common serologic test requests.
Sample Volume (Tube) Requirement:
- One full draw 5mL SST tube for a combination of any 6 assays listed below.
- Two full draw 5mL SST tubes for a combination of more than 6 assays listed below.
Serology Test Options
| CMV IgG |
| CMV IgM |
| EBV IgG |
| EBV IgM |
| Hepatitis A IgG |
| Hepatitis A IgM |
| Hepatitis B Surface Antigen |
| Hepatitis B Core Antibody (Total) |
| Hepatitis B Surface Antibody |
| Hepatitis B Core IgM |
| Hepatitis B e Antibody |
| Hepatitis B e Antigen |
| Hepatitis C Antibody (HCV) |
| HIV |
| HSV I |
| HSV II |
| HTLV |
| Measles IgG |
| Measles IgM |
| Mumps IgG |
| Mumps IgM |
| Parvovirus IgG |
| Parvovirus IgM |
| Rubella IgG |
| Rubella IgM |
| Syphilis |
| Toxoplasmosis gondii IgG |
| Toxoplasmosis gondii IgM |
| Varicella zoster IgG |
| Varicella zoster IgM |
* If there is a high clinical suspicion that the patient has an HCV infection and testing for other serological markers is needed, consider submitting two SST to ensure there is adequate volume.
For all other serological assays, special handling instructions and detailed information visit the Test Information Index and navigate to the specific test.
Use serum separator tubes (SST),which are optimized for PHO’s Total Laboratory Automation (TLA) system
PHO implemented a TLA system for serology testing, which comprises pre-analytic, analytic and post-analytic components. To optimize this automated track system and improve turnaround times for testing, PHO laboratory prefers that clients submit centrifuged SST for serological testing requests. Submission of centrifuged SST improves the assessment of specimen quality (e.g. presence of hemolysis and/or lipemia, etc.) and accelerates specimen processing and testing as it is most compatible with the track system.
The receipt of tube types that have not been optimized and/or validated by PHO (e.g. rubber stopper and Cryovials) require manual processing which delays the turnaround time for testing. The automated track system does NOT accept rubber stopper tubes. See images below for preferred submission tubes.

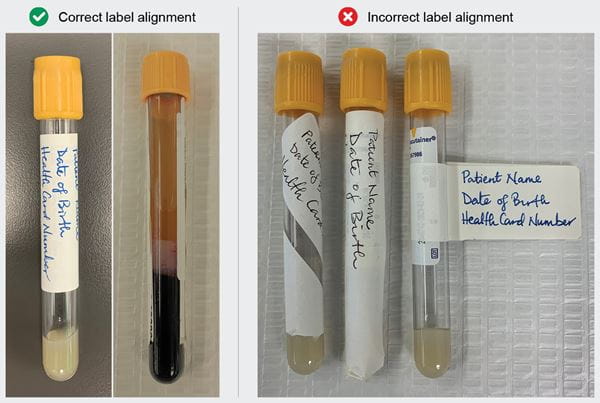
Correctly Affix Patient Labels on Tubes
Affix the patient label vertically along the specimen tubes, leaving a gap so that specimen integrity can be assessed. Improper labelling creates safety issues, delays processing time and testing, which may impact patient care. See images below for examples of correct and incorrect label alignments.
Don’t have a MyPHO account? Register Now

