Hepatitis B – Serology
Consistent with O. Reg. 671/92 of the French Language Services Act, laboratory testing information on this page is only available in English because it is scientific or technical in nature and is for use only by qualified health care providers and not by members of the public.
This page provides hepatitis B virus (HBV) serology testing information at Public Health Ontario (PHO). Information is specific to HBV serology. For information regarding other testing options for HBV, refer to the following PHO webpages:
Updates:
- Updated the Acceptance/Rejection Criteria section as follows:
- Cadaveric blood testing services are not available through PHO. Requests can be submitted to Mt. Sinai Hospital Microbiology Laboratory at 416-586-4800 extension 4432.
- Specimens with mismatched information on the label and requisition will be rejected.
- Updated submission and collection notes to outline ordering instructions for HBV serology.
Testing Indications
Testing for HBV serology may be indicated in the following:
- suspected acute or chronic hepatitis
- pre-natal screening
- determining the immune status (either following recovery from natural infection or as a result of immunization)
- prior to starting chemotherapy
- occupational and other exposures
- investigation of known or suspected infection control breaches
- outbreak situations
- hepatitis B virus reactivation (HBVr) in those who are chronic carriers
- immune status of at-risk patients to HBVr
If ordering as part of prenatal screening, see Prenatal – Serology.
Acceptance/Rejection Criteria
- Cadaveric blood testing services are not available through PHO. Requests can be submitted to Mt. Sinai Hospital Microbiology Laboratory at 416-586-4800 extension 4432.
- Donor testing is not available through PHO. Specimens from patients being screened as potential donors (e.g. organ, tissue, cells, fertility, etc.) should be referred to a laboratory that performs donor screening assays. Specimens received for donor screening at PHO will be rejected. Specimens with mismatched information on the label and requisition will be rejected.
Specimen Requirements
| Test Requested | Required Requisition(s) | Specimen Type | Minimum Volume | Collection Kit |
Hepatitis B Serology |
Serum |
1.5 mL serum |
Vacutainer tubes – Serum Separator Tube (SST); Gold top |
Submission and Collection Notes
Complete all fields of the requisition form, including:
- Test(s) requests (see Special Instructions) and reason for test
- Patient setting and specimen type and site
- Relevant clinical information
Label the specimen tube with the patient’s full name, date of collection and one other unique identifier such as the patient’s health card number or date of birth. For additional information see: Criteria for Acceptance of Patient Specimens. Failure to provide this information may result in rejection or testing delay.
Refer to HBV Serology Test Ordering Instructions below for how to complete the Hepatitis Serology section of the general test requisition.
One FULL 5 mL SST is required for testing a combination of hepatitis virus, HIV, HTLV, syphilis and rubella.
Do NOT submit glass tubes.
Limitations
Heat inactivated, haemolysed, icteric, lipemic or microbially contaminated serum is not recommended for testing.
Storage and Transport
Place specimen tube in biohazard bag and seal it. Place completed General Test Requisition in the pouch at the front of the biohazard bag.
To avoid delays in testing, ship specimens for testing to PHO immediately after collection or processing. Store specimen tubes at 2-8°C following collection and centrifugation, and ship to PHO’s laboratory on ice packs. If delayed shipping is anticipated, remove serum from clot and store frozen at -20°C or colder, and ship on dry ice.
All clinical specimens must be shipped in accordance to the Transportation of Dangerous Good Act.
Special Instructions
HBV serology ordering instructions
| Test(s) Requested on Requisition | Test(s) Performed | Notes | Requisition Example |
|---|---|---|---|
|
Chronic Infection |
HBsAg, HBcAb Total (IgG+IgM) |
|
|
|
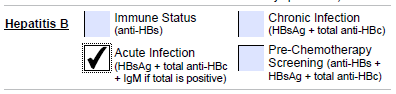
Acute Infection |
HBsAg, HBcAb Total (IgG+IgM)
|
HBcIgM will be performed only if HBcAb Total (IgG+IgM) is reactive |
|
|
Immune Status |
Anti-HBs |
|
|
|
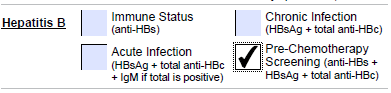
Pre-chemotherapy screening
|
HBsAg, HBcAb Total (IgG+IgM), Anti-HBs |
For cancer patients prior to commencing systemic therapy (chemotherapy, targeted therapy, immunotherapy) |
|
|
Chronic and Immunity |
HBsAg, HBcAb Total (IgG+IgM), Anti-HBs |
A reason for testing must be indicated under Testing Indication(s) / Criteria (e.g. Dialysis) |
|
|
Acute and Immunity |
HBsAg, HBcAb Total (IgG+IgM), Anti-HBs |
A reason for testing must be indicated under Testing Indications(s) / Criteria (e.g. Dialysis)
HBcIgM will be performed only if HBcAb Total (IgG+IgM) is reactive |
|
|
Chronic Infection
and
Test(s) Requested: HBeAg, HBeAb |
HBsAg HBcAb Total (IgG+IgM)
|
Testing for HBeAg and HBeAb will only be performed if HBsAg is reactive |
|
|
Acute Infection and Test(s) Requested: HBeAg, HBeAb
|
HBsAg HBcAb Total (IgG+IgM)
|
Testing for HBeAg and HBeAb will only be performed if HBsAg is reactive |
|
|
Test(s) Requested: HBsAg, Anti-HBS |
HBsAg Anti-HBS |
Do not complete the Hepatitis Serology section. Enter the specific test(s) under Test(s) Requested. Under Testing Indication(s) / Criteria, specify “Hepatitis B Exposure” under Other |
|
|
Test(s) Requested: HBeAg and HBeAb or HBcIgM |
HBeAg/HBeAb or HBcIgM |
Do not complete the Hepatitis Serology section. Enter the specific test(s) under Test(s) Requested |
|
Test Frequency and Turnaround Time (TAT)
HBV serology testing is performed daily Monday to Friday.
Turnaround time may be up to 3 business days from receipt of the specimen by PHO’s laboratory for “non-reactive” specimens and up to 6 business days for “reactive” specimens.
STAT and Critical Samples Testing
If warranted, testing of the ‘source patient’ for needle stick/occupational exposures and high risk patients presenting in labour who have not received prenatal care or for whom results of previous testing are not readily available are expedited.
Prior to sending, PHO’s Customer Service Centre must be notified at 416-235-6556/1-877-604-4567. Contact the After-Hours Emergency Duty Officer at 416-605-3113 if outside of PHO’s laboratory operating hours.
“STAT” specimens must be shipped separately from routine specimens. The outer packaging must clear be marked “STAT” and handled in accordance with the Canadian Biosafety Standards. Specimens must be shipped in accordance with the Transportation of Dangerous Goods Regulations. Failure to ship separately, specimen will be tested as routine.
Specimens submitted for HBV serology are tested using a chemiluminescent microparticle immunoassay (CMIA) for the qualitative detection of HBV markers (either antibodies or antigens).
Algorithm
Refer to the table under HBV Serology Test Ordering Instructions for the tests that will be performed based on the request.
If HBsAg is reactive, but HBcAb Total is non-reactive then the specimen will be tested using an HBsAg confirmatory test (‘blocking assay’).
Interpretation
Please refer to CDC Resources for interpretation of HBV serology test results.
Reporting
Results are reported to the ordering physician, authorized health care provider (General O. Reg 45/22, s.18), or submitter as indicated on the requisition.
Specimens that are positive for HBV surface antigen (HBsAg) are reported to the Medical Officer of Health as per the Ontario Health Protection and Promotion Act.
Don’t have a MyPHO account? Register Now