
Avian Influenza and Influenza A Subtyping – Real-time PCR
Consistent with O. Reg. 671/92 of the French Language Services Act, laboratory testing information on this page is only available in English because it is scientific or technical in nature and is for use only by qualified health care providers and not by members of the public.
Background
This page provides molecular testing information for Influenza A subtyping and avian influenza at Public Health Ontario (PHO). For information regarding other testing options, refer to the following PHO webpages:
Updates
- Starting December 12, 2025, PHO’s laboratory will perform subtyping analysis on a portion of specimens positive for influenza A that could not be subtyped by other means. If Influenza A (not subtyped) is detected in a health unit declared outbreak, one specimen with a high viral load (Ct < 30) will be selected for subtyping.
- Subtyping for surveillance will be limited to specimens received by PHO’s laboratory on Thursdays only.
- Updated the title of the document to ‘Avian Influenza and Influenza A Subtyping’
Testing Indications
Avian Influenza
PHO is responding to increase in the number of cases of highly pathogenic avian influenza (H5N1) in birds across Ontario. In addition, H5N1 was previously detected in dairy cows in several farms in the United States with confirmed human cases in dairy and poultry workers. The investigation number ONT-2022-00001 should be included on all requisitions for local exposures related to infected birds or other animals. Testing for symptomatic individuals will include COVID-19, avian influenza and multiplex respiratory virus PCR.
*Community and hospital laboratories that perform novel influenza or non-seasonal influenza testing (including H5) should send positive specimens to PHO for further analysis and specimen will also be forwarded to the National Microbiology Laboratory (NML) for confirmatory testing.
Results are considered preliminary until confirmed by the NML.
Contact your local public health unit if there is a suspected avian Influenza infection.
Influenza A Subtyping
To support preparedness and response for the 2025-2026 respiratory virus season in Ontario, PHO will continue to subtype Influenza A positive specimens which will be selected based on specific testing criteria. The enhanced influenza surveillance program is essential for early identification of novel influenza strains including the Highly Pathogenic Avian Influenza (HPAI) H5N1.
Effective December 12, 2025, the following criteria will guide the selection of positive influenza specimen for submission for Influenza A subtyping or whole genome sequencing under PHO’s enhanced influenza surveillance program:
- Specimens from hospitalized (ward) patients that are positive for influenza A with sufficiently high viral load (equivalent to Ct<30) and cannot be subtyped by the primary testing laboratory using their current commercial or lab developed test (LDT) PCR assays (i.e., non-H3/non-H1 specimens). Laboratories are encouraged to submit only one full day’s worth of positive specimens every week. Only specimens received at PHO’s laboratory sites on Thursday will be tested.
- Specimens from all cases of suspected animal-to-human transmission of influenza A virus
- Specimens from all patients with suspected influenza anti-viral resistance
- Specimens from all ICU patients regardless of typing results
- Specimens positive for Influenza A from a health unit declared outbreak that could not be subtyped by other means such as the Multiplex Respiratory Virus PCR. One specimen with high viral load will be subtyped.
Acceptance/Rejection Criteria
Serology testing is not recommended for influenza diagnosis, including avian influenza, and is not available for clinical testing in Canada.
Specimen Requirements
| Test Requested | Required Requisition(s) | Specimen Type | Minimum Volume | Collection Kit |
Avian Influenza |
Nasopharyngeal swab, AND throat swab |
1 swab in the collection kit media for each collection site |
Nasopharyngeal Collection Kit for Viral PCR order#: 390082 Large Swab Throat Collection Kit for Viral PCR order#: 390081 |
|
Avian Influenza |
Bronchoalveolar Lavage (BAL), bronchial wash (BW) or pleural fluid |
2.0 ml |
Sterile container |
|
Avian Influenza |
Respiratory tract tissue |
1.0 g |
Sterile container |
|
Avian Influenza |
Conjunctival swab (Do not submit until approved by a PHO microbiologist) |
1 swab in the collection kit media (UTM) |
||
Influenza A Subtyping |
Influenza A positive Primary sample |
0.5 ml |
Submission and Collection Notes
Label the specimen container(s) with the patient’s first and last name, date of collection, and one other unique identifier such as the patient’s date of birth or health card number. Failure to provide this information may result in rejection or testing delay.
Complete all fields of the PHO’s General Test Requisition Form, including:
- Tests requested and reason for test
- Patient setting, specimen types and sites
- Travel history, exposure history and clinical information
- Ct value for Influenza A if available
Serology testing is not recommended for influenza diagnosis, including avian influenza, and is not available for clinical testing in Canada.
For Avian Influenza testing:
- Prior to specimen submission for Avian Influenza testing, contact PHO’s Laboratory Customer Service Centre at 416-235-6556 or 1-877-604-4567 or after-hours Emergency Duty Officer at 416-605-3113 for further instructions and testing approval. Weekend testing is available and must be approved by a PHO microbiologist or designate.
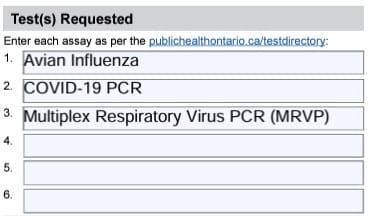
- Under Tests Requested include all the following Avian Influenza, COVID-19 PCR, and Multiplex Respiratory Virus PCR/MRVP
- In the Outbreak field of the requisition include ONT-2022-00001 (see special instruction below)
- Upper respiratory tract samples, including nasopharyngeal (NP) swab and throat swab in universal or viral transport medium (UTM/VTM) are recommended for all symptomatic patients being investigated for avian influenza.
- Lower respiratory tract samples - e.g. bronchoalveolar lavage (BAL), bronchial wash, and/or lung tissue if obtained (e.g. biopsy, post-mortem) are recommended for severe cases with lower respiratory tract infection
- Sample types other than respiratory, such as swabs from other potentially infected anatomical sites (e.g. conjunctival swabs from patients with conjunctivitis and potential occupational exposure), require approval by a PHO microbiologist.
- Stool and blood are not routinely tested for avian influenza but may be tested if avian influenza is detected in respiratory specimens. Contact PHO for approval by a PHO microbiologist before you submit these specimens.
For Influenza A Subtyping:
- Under Tests Requested indicate Influenza A Subtyping
- Include the Ct value for Influenza A if available
- A minimum of 0.5ml of the primary specimen is required for influenza A subtyping
- If specimens are sent for surveillance testing, ensure they will be received by PHO's laboratory on Thursday.
Storage and Transport
Place specimen in biohazard bag and seal. Specimens should be stored at 2-8°C following collection. Package and ship primary clinical samples to the local PHO location in accordance with the Transportation of Dangerous Goods Regulations.
Special Instructions
For Avian Influenza testing requests (following exposure to potentially infected animals):
Complete all fields of the PHO General Test Requisition Form.
Under Test(s) Requested, order avian influenza, COVID-19 PCR and multiplex respiratory virus PCR (MRVP) (See example below)
Travel history and clinical information must be provided. Indicate the following: travel to farms or areas experiencing avian influenza activity; symptoms and clinical conditions including pneumonia ; high risk status for respiratory viral infection complications; patient setting; admission status at time of collection (if known).
Test Frequency and Turnaround Time (TAT)
|
Test |
Turnaround Time |
Test Location |
Frequency |
|---|---|---|---|
|
Avian Influenza subtyping - H5 target only 1 |
Up to 2 business day(s) from specimen receipt at PHO |
Ottawa |
Monday to Friday |
|
Avian Influenza subtyping – full panel |
Up to 2 business day(s) from specimen receipt at PHO |
Toronto |
Monday to Friday |
|
Influenza A Subtyping (routine specimens) |
Up to 5 business day(s) from specimen receipt at PHO |
Toronto |
Monday to Friday |
|
Influenza A Subtyping (patient hospitalized in ICU or animal exposure and one patient from an outbreak) |
Up to 2 business day(s) from specimen receipt at PHO |
Toronto |
Monday to Friday |
1 Specimen will be forwarded to Toronto for full panel typing after H5 typing is completed at one of the regional testing laboratories.
Positive avian influenza (H5, H7, or N9) specimens will be forwarded to the National Microbiology Laboratory (NML) for confirmation. TAT to receive results is up to 14 days from receipt of specimens at the NML.
STAT and Critical Specimens Testing
STAT testing is not available.
Priority avian influenza testing is available outside of regular hours upon approval from a PHO microbiologist for symptomatic individuals (all patient settings) with potential exposure to poultry, wild birds, livestock, or other animals. Please contact PHO’s Laboratory Customer Service at 416-235-6556/1-877-604-4567 or after-hours Duty Officer at 416-605-3113 for consultation.
Priority avian influenza specimens must be shipped separately from routine specimens with the outer package clearly labelled ‘priority’. It must be handled in accordance with the Canadian Biosafety Standards and shipped in accordance with the Transportation of Dangerous Goods Regulations. Failure to ship separately will delay testing.
Influenza A and B are tested with CDC in-house Real Time PCR assay.
Influenza A positive specimens will be subtyped with Real time RT-PCR.
Algorithm
Specimens received in Toronto, will be initially tested for Influenza A and B. Influenza A positive specimens will be further tested for full Avian Influenza panel typing (H1N1pdm09, Human H3, avian H5, avian H7, and N9).
Specimens received in one of the designated regional laboratories, will be initially screened for H5 and will be forwarded to Toronto for full Avian Influenza panel typing.
Positive avian influenza (H5, H7, or N9) specimens will be forwarded to NML for confirmation.
Reporting
Results are reported to the physician, authorized health care provider (General O. Reg 45/22, s.18), or submitter as indicated on the requisition.
Specimens that are positive for avian Influenza are reported to the Medical Officer of Health as per Health Protection and Promotion Act.
Don’t have a MyPHO account? Register Now

