
HIV – Diagnostic Serology
Consistent with O. Reg. 671/92 of the French Language Services Act, laboratory testing information on this page is only available in English because it is scientific or technical in nature and is for use only by qualified health care providers and not by members of the public.
Background
This page provides diagnostic serology testing information for human immunodeficiency virus (HIV), the causative agent of acquired immunodeficiency syndrome (AIDS), at Public Health Ontario (PHO). For information regarding other testing options, refer to the following PHO webpages:
- HIV-1/-2 PCR
- HIV-1 Genotyping, Resistance, Tropism and HLA-B*57:01 Abacavir Hypersensitivity Testing
- HIV-1 RNA Viral Load
- Prenatal – Serology
Updates
- Acceptance/Rejection Criteria:
- Cadaveric blood testing services are not available through PHO. Requests can be submitted to Mt. Sinai Hospital Microbiology Laboratory at 416-586-4800 extension 4432.
- Mismatched information on the specimen tube label and requisition will be rejected.
- Reporting: Added information regarding LEP Questionnaire and CATIE Information Sheet attachments to all first-time HIV positive reports.
- Timing of Specimen Collection: Added information pertaining to the timing of specimen collection and HIV reactivity.
Testing Indications
HIV testing should be considered as part of routine care. Specifically, testing is recommended for individuals:
- with symptoms of acute HIV infection
- with symptoms associated with chronic HIV infection
- who have had a high-risk exposure
- who belong to a population with higher rates of HIV
- who are initiating immune suppressing therapy
- who are pregnant; if ordering as part of a prenatal screening, see Prenatal – Serology
For more information on HIV testing recommendations, refer to the Ontario Guidelines for Providers Offering HIV Testing.
Acceptance/Rejection Criteria
- Cadaveric blood testing services are not available through PHO. Requests can be submitted to Mt. Sinai Hospital Microbiology Laboratory at 416-586-4800 extension 4432.
- Donor testing is not available at PHO. Specimens from patients being screened as potential donors (e.g. organ, tissue, cells, fertility, etc.) should be referred to a laboratory that performs donor screening assays. Specimens submitted for donor screening to PHO will be rejected.
- Haemolysed, icteric, lipemic or microbially contaminated sera are unacceptable for testing.
- Mismatched information on the specimen tube label and requisition will be rejected.
Specimen Requirements
| Test Requested | Required Requisition(s) | Specimen Type | Minimum Volume | Collection Kit |
HIV Serology |
Serum |
1.5 ml serum |
Serum Separator Tube (SST) |
Submission and Collection Notes
Specimens may be stored at 2-25°C following collection. Centrifugation should be performed according to the tube manufacturer’s instructions, ideally within 24 hours from collection. Spun SST can be stored at 2-8°C for up to 5 days.
Complete all fields of HIV Serology and PCR requisition.
Label the specimen tube(s) with the patient’s first and last name, date of collection, and one other unique identifier such as the patient’s date of birth or health card number. For additional information, see Criteria for Acceptance of Patient Specimens. Failure to provide this information may result in rejection or testing delay. Patient information on the specimen must match the requisition.
Generally, only one FULL draw of 5 mL SST is needed to test a combination of up to six infectious disease serology markers. If a full tube cannot be drawn, submit two tubes. Refer to Serology Testing – Laboratory Specimen Collection and Submission Instructions for more information.
Do not submit glass tubes.
Timing of Specimen Collection
PHO uses a 4th generation HIV Ag/Ab screening test. In general, individuals will test reactive 2-4 weeks after exposure (median 18 days) and >99% of individuals will be reactive by 6 weeks.1
Storage and Transport
Place the specimen tube in a biohazard bag and seal it. Place completed HIV Serology and PCR Test Requisition in the pouch at the front of the biohazard bag.
To avoid delays in testing, ship specimens for testing to PHO’s laboratory immediately after collection or processing. Spun SST or separated serum stored at 2-8°C must be shipped with ice packs to PHO’s laboratory. If delayed shipping is anticipated, remove serum from clot and store frozen at -20°C or colder, and ship on dry ice.
All clinical specimens must be shipped in accordance with the Transportation of Dangerous Good Act.
Test Frequency and Turnaround Time (TAT)
HIV diagnostic serology testing is performed daily Monday to Friday.
Turnaround time is up to 3 business days for non-reactive specimens and up to 6 business days for reactive specimens from receipt by PHO’s laboratory.
Stat and Critical Samples Testing
STAT testing should only be requested when it directly affects patient care in an emergency medical circumstance. If warranted, the following HIV testing is expedited:
- Testing of the ‘source patient’ for needlestick/occupational exposure.
- A pregnant individual presenting in labour who has not received prenatal care or for whom results of previous testing are not readily available and are at high risk for HIV.
After-hours STAT testing requires approval . Contact the after-hours Duty Officer at 416-605-3113 if outside of PHO’s laboratory operating hours.
PHO uses the following methods for HIV diagnostic testing:
- HIV-1/-2 antigen (Ag)/antibody (Ab) combination screen: Chemiluminescent microparticle immunoassay (CMIA) that simultaneously detects HIV-1 p24 Ag and HIV-1 and HIV-2 Ab. This assay does not differentiate between p24 and HIV-1/-2 Ab.
- HIV-1/-2 antibody confirmatory test: Geenius™ immunochromatographic test for confirmation and differentiation of antibodies to HIV-1 and HIV-2.
- HIV-1/-2 nucleic acid test (NAT): Qualitative NAT for the detection and differentiation of HIV-1 and HIV-2 RNA.
Algorithm
Serum specimens submitted for HIV serology are tested following the algorithm below:
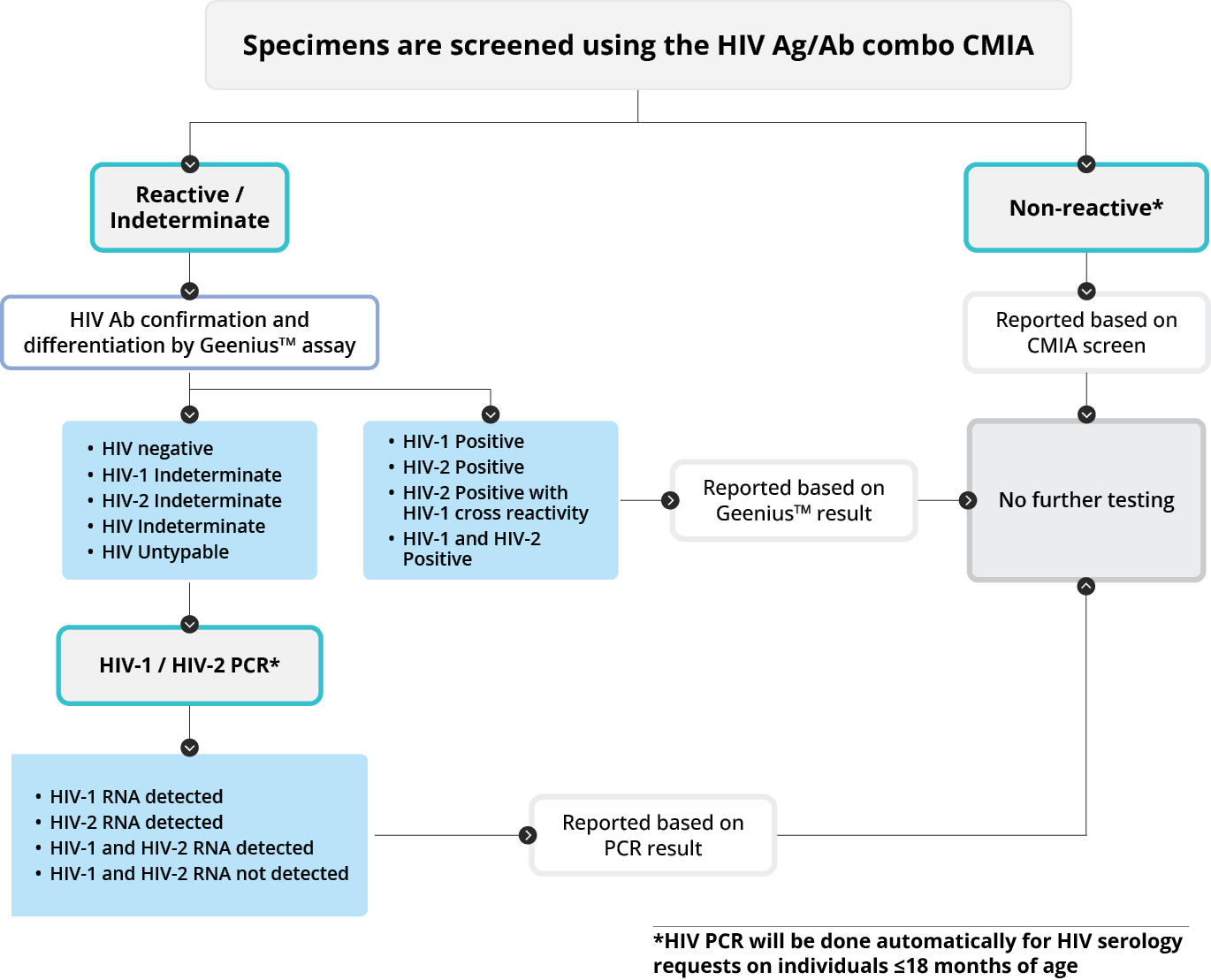
Note: To help determine HIV status and guide treatment decisions, HIV PCR will be prioritized over HIV serology for individuals ≤18 months of age. If there is a high suspicion of acute HIV in the window period (i.e. prior to seroconversion), consider requesting both HIV serology and HIV PCR testing.
Interpretation
Results should be interpreted in the context of the patient’s clinical presentation, risk factors, and exposure history, and treatment. The table below provides a general overview of the algorithm and the most common interpretations for HIV diagnostic testing for adults. It does not include all possible result scenarios. These interpretations may not apply to infants as maternal antibodies can circulate for 18-24 months after birth. Consider referral to a paediatric HIV specialist for management of infants suspected of HIV infection.
|
HIV-1/HIV-2 Ag/Ab Combo Screen |
HIV-1/HIV-2 Ab Confirmatory |
HIV-1/HIV-2 PCR |
Interpretation |
|---|---|---|---|
|
Non-reactive |
Not tested |
Not tested |
Non-reactive for HIV-1 p24 antigen and HIV-1/-2 antibodies. For high-risk exposures, repeat HIV testing at 3 weeks and, if negative, at 6 weeks after possible exposure or on presentation of symptoms compatible with acute HIV infection. If there is a high suspicion of acute HIV in the window period (i.e. prior to seroconversion), consider requesting HIV PCR testing. |
|
Reactive/ Indeterminate |
Negative/ Indeterminate |
HIV-1 and HIV-2 RNA not detected |
Inconclusive for HIV infection.
Repeat testing in at least 4 weeks from date of collection. If two or more inconclusive results have been obtained on separate specimens, no further testing for HIV is recommended unless clinically warranted. |
|
Reactive/ Indeterminate
|
HIV-1 positive |
Not tested |
Evidence of HIV-1 infection. |
|
Reactive/ Indeterminate |
HIV-2 positive |
Not tested |
Evidence of HIV-2 infection. |
|
Reactive/ Indeterminate |
HIV-1 and HIV-2 positive |
Not tested |
Evidence of HIV-1 and HIV-2 co-infection. |
|
Reactive/ Indeterminate |
Negative/ Indeterminate |
HIV-1 RNA detected |
Evidence of acute HIV-1 infection. |
|
Reactive/ Indeterminate |
Negative/ Indeterminate |
HIV-2 RNA detected |
Evidence of acute HIV-2 infection. |
|
Reactive/ Indeterminate |
Negative/ Indeterminate |
HIV-1 and HIV-2 RNA detected |
Evidence of acute HIV-1 and HIV-2 co-infection. |
A PHO Microbiologist is available for questions about HIV serology results and interpretations by calling 416-235-6556 / 1-877-604-4567 or by email at CustomerServiceCentre@oahpp.ca.
For individuals who test positive for HIV-1, consider submitting a follow-up EDTA whole blood or plasma specimen for HIV-1 viral load testing. Refer to the HIV-1 Viral Load Test Information Sheet for detailed information.
Reporting
Results are reported to the physician, authorized health care provider (General O. Reg 45/22, s.18) or submitter as indicated on the requisition.
The PHO Laboratory Enhancement Program (LEP) questionnaire and a CATIE letter will accompany all first-time HIV-positive results .
- LEP Questionnaire: A voluntary questionnaire used to gather patient demographics (e.g., race/ethnicity, country of birth), HIV risk factors, and testing/treatment history. This data is essential for provincial HIV surveillance in Ontario and informs policy and programming decisions.
- CATIE Information Sheet: An information sheet that includes resources to help newly diagnosed individuals access treatment, understand the role of public health in follow-up and contact notification, and offers strategies to prevent transmission, along with resources for support services.
Specimens that are positive for HIV diagnostic testing are reported to the Medical Officer of Health as per the Ontario Health Protection and Promotion Act.
Due to changes in PHO’s and Ontario’s laboratory information systems, cumulative reporting is no longer available.
Don’t have a MyPHO account? Register Now

